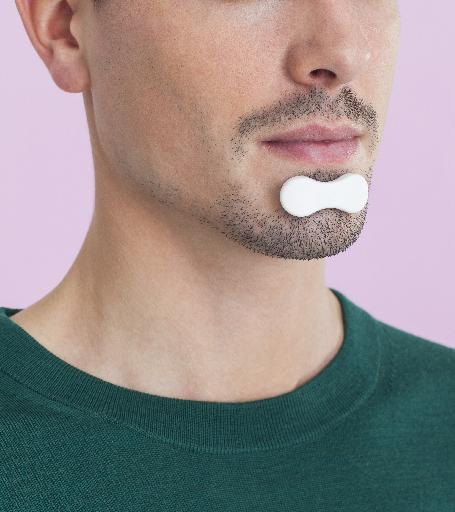
The Belgian start-up Sunrise, is launching a medical device that facilitates the diagnosis of sleep disorders.
The device is the result of over 10 years of research. It demonstrates a correlation between sleep disordered breathing and chin movements. Placed on the chin, before going to sleep, it analyses the quality of sleep.
The start-up recently raised 1 million euros, and collaborates with several academic research centres.
Among current sleep disorders, insomnia is the most widespread, followed by sleep apnoea which remains to be hugely undetected, Sunrise explained.
Nearly one in two men and one in four women older than 40 often snor. It is complicated to get a medical diagnosis, and the diagnosis rate remains therefore very low (only 20%) of a disorder that triples the risk of death.
Sleep disordered breathing entails significant risks for undiagnosed patients: strokes, cardiac arrhythmia, diabetes, hypertension or obesity, Sunrise underlined.
In addition, an undiagnosed patient costs society an average of 6,000 euros per year, compared to 2,000 euros for a diagnosed and treated individual.
Sunrise has invented a light - 3 grams - sensor that uses the latest software and hardware technologies for a simple and reliable sleep diagnosis.
The patient can purchase the device himself, connect it to a mobile application and paste it on his chin overnight.
The next morning, a complete sleep analysis is obtained. The person can then also share the full report with his physician.
The sensor has been widely clinically tested amongst a sample of 400 persons and has demonstrated unprecedented reliability, affirms Sunrise.
The scientific community and medical practitioners believe that this is the most economical and convenient way to diagnose the sleep apnoea syndrome.
The patented technology has obtained the CE marking and is thus a certified medical device subject to inspection by a notified EU body. It is now pending approval of the US Food and Drug Administration (FDA).
The Brussels Times