The US Food and Drug Administration (FDA) has given the green light for a new drug against Alzheimer’s disease.
The drug, Lecanemab, is the first treatment proven to slow the advance of the disease. It was developed by pharmaceutical companies Eisai and Biogen and received accelerated approval by the FDA.
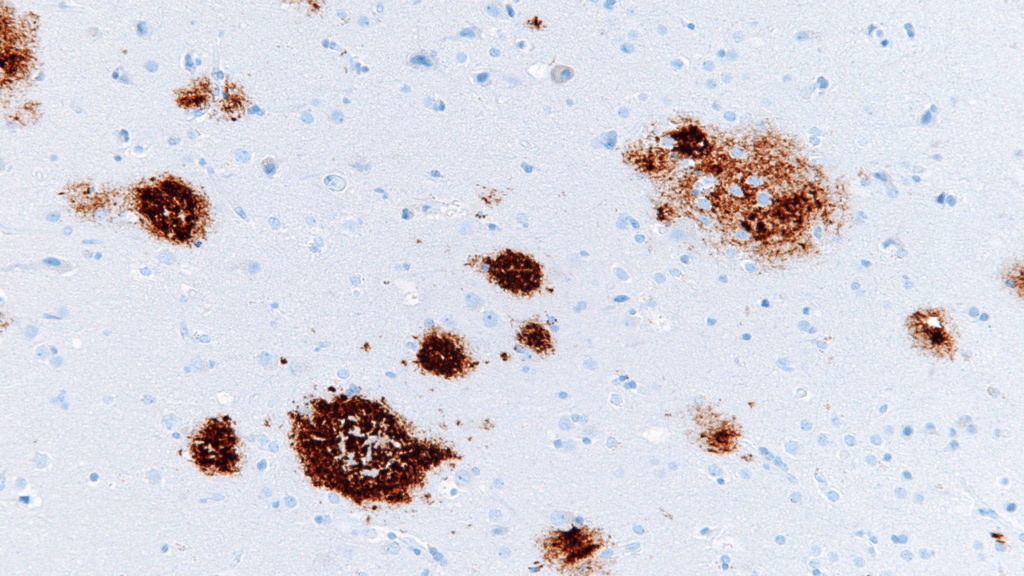
Lecanemab, which will be sold under the brand name Leqembi, does not cure Alzheimer’s disease but slows its advance by acting on the amyloid proteins that underlie the most common form of dementia.
Trials have shown that over an 18-month period, the gene agent slows cognitive decline by 27%.