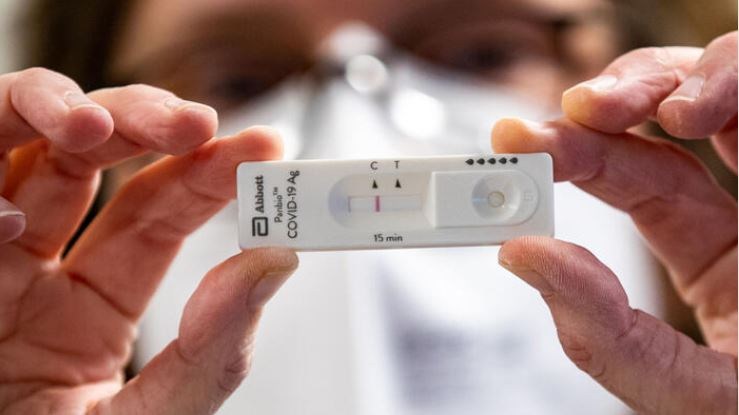
Belgium's Federal Agency for Medicines and Health Products (FAMHP) has approved a second coronavirus rapid test that can soon be sold as a self-test.
In addition to the rapid tests developed by Roche, a Biosynex rapid test will also be available from pharmacies from 6 April as part of Belgium's "testing strategy 2.0."
These self-tests are rapid antigen tests that are both taken and interpreted by the user, with the result available in 15 to 20 minutes.
However, as there are currently no tests on the European market that have received a CE-marking, only tests for professional use that meet strict requirements and have been verified by the FAMHP may be sold to private individuals, and only via pharmacists.
Related News
- Cheat Sheet: Belgium's 'testing strategy 2.0'
- Self-tests should not be used as free pass for concerts or to travel, Belgian microbiologist says
- Why Belgium only sells self-tests in pharmacies, while Germans buy them in supermarkets
Additionally, the pharmacist is required to inform the buyer about the test's use and must also make it clear that consulting with a doctor is mandatory after a positive result.
For people who are entitled to an increased allowance, the tests will cost €1. For the general population, the price will vary between €7 and €8, depending on the test, according to the authorities.
Additionally, the FAMHP announced that a table on its website will systematically be updated with the tests that have been approved for use by the agency.
Maïthé Chini
The Brussels Times