The rollout of the vaccination programmes in EU became more transparent this week when the European Centre for Disease Prevention and Control uploaded a special tracker mechanism to monitor the deliveries and use of the vaccines and published an updated overview of the national vaccination rollout across the EU.
As previously reported, the COVID-19 Vaccine Tracker is an interactive ‘live’ dashboard that provides the latest data reported by EU/EEA countries. The data are updated biweekly and are subject to retrospective corrections. The ECDC added a disclaimer and warned users to use data with caution.
The ECDC overview presents some new insights into some of the critical aspects and challenges member states are experiencing with the implementation of national deployment plans in the EU/EEA and summaries the figures in the Vaccine Tracker.
In most countries, the vaccination campaigns started between the 26 and 31 December 2020, shortly after the first lots of vaccines (Pfizer/BioNTech) were delivered to all EU/EEA countries by the manufacturer. In addition to Pfizeer/BioNTech, by the 28 January, at least 22 countries reported having started administering the Moderna vaccine.
Challenges countries are facing with the rollout of the vaccines include, among others: shortage of equipment, in particular a lack of low dead space syringes and needles; communication challenges and the spread of disinformation; challenges with monitoring systems such as consolidating quality of registry data, logistical challenges and limited vaccine supply.
Besides the health authorities in the member states, there are three EU bodies that are supposed to provide all the answers on the vaccinations programmes: the European Commission, the European Medicines Agency (EMA) and the European Centre for Disease Prevention and Control (ECDC).
The Brussels Times asked them some questions and publish here their answers.
The vaccines are supposed to be distributed to the member states on a pro-rata basis in relation to their populations - does it follow from the figures?
A table (3) in the ECDC overview shows that the proportion of distributed vaccines per hundred inhabitants (18+) vary somewhat between the member states, from 3,5 in Spain to 6,1 in Denmark (Malta is an outlier with 9,8). No explanation is given to the variation.
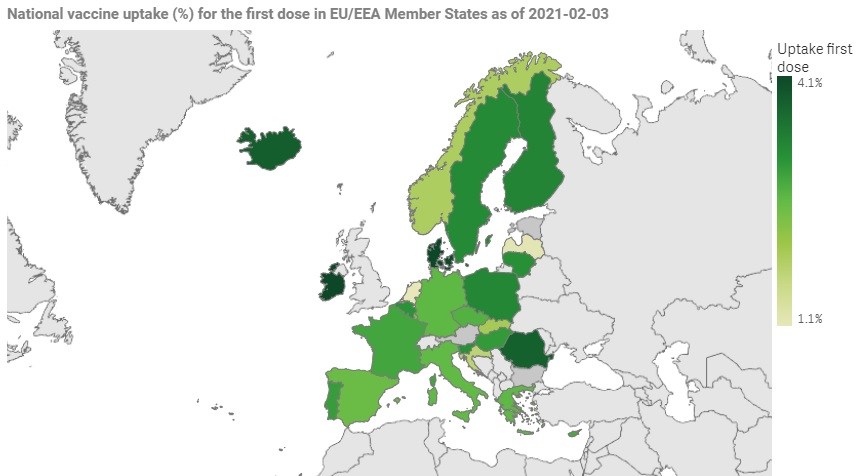
© ECDC
The estimate of the national vaccine uptake for the first dose among adults (18+) varies between 0.9% and 3.8%. Does the variation indicate differences in the effectiveness of the vaccination rollout?
No clear reply was given to this question. ECDC comments that the proportion of distributed doses administered may be affected by the country decision to delay the second dose to deliver the first dose to the highest possible number of individuals versus to ensure availability of the complete course of two doses to those that have received the first dose.
Several countries have decided not to extend the timing between the first and second doses following the recommendation from the vaccine producers (at least 21 days for Pfizer-BioNTech and 28 days for Moderna). Most countries have replied that for the time being they will not extend the timing between the first and second dose (14 countries), or that the decision is still pending (six countries).
Available information seems to indicate that the second dose of the Pfizer vaccine should be given between 21 and 28 days after the first dose. Two countries have extended the interval. The Netherlands, for example, has decided to extend the dose interval for Pfizer/BioNTech to up to 42 days, i.e. double the recommended minimum interval of 21 days.
Is the Commission in favour of extending the timing between the two doses to vaccinate as many people as possible with one dose until more doses will be available?
This is a question for EMA which however did not reply to a request for clarification about the timing. ECDC refers to WHO’s recommendation that based on currently available clinical trial data the interval between vaccine doses may be extended up to 42 days (six weeks).
Is it possible to forecast the vaccine deliveries in the coming months?
According to a source in the Commission, even without AstraZeneca, more than 500 million doses from the Pfizer- BioNTech and Moderna vaccines can be delivered by end of September.
“If we include the Johnson & Johnson vaccine that number is 600 million. The approval of the Johnson and Johnson vaccine could be given by mid-February, and as a ‘single shot’ vaccine, risks associated with the administration of a second dose (missed appointments), as for other approved vaccines, are removed.”
AstraZeneca announced recently that will deliver 40 million doses in total, 9 million additional doses for the first quarter as compared to last week’s offer, and this one week earlier than scheduled. They will also expand their manufacturing capacity in Europe. Pfizer will also normalize its delivery from mid-February and step up significantly once the Marburg plant is approved by EMA in the coming weeks.
EMA approved the AstraZeneca vaccine for use in in people from 18 years of age. According to EMA, the vaccine has demonstrated around a 60% efficacy in the clinical trials.
“Whilst most of the participants in these studies were between 18 and 55 years old and there are not yet enough results in older participants to provide a figure for how well the vaccine will work in this group, protection is expected,” according to EMA. However, some countries have already decided not to administer it to people over 65. The latest to announce this were Belgium and Sweden.
According to the Commission’s targets, at least 80% of people over the age of 80 and 80% of health and social care professionals in every member state should be vaccinated by March 2021. In addition, a minimum of 70% of the adult population should be vaccinated by summer 2021. Will EU achieve its vaccination goals?
The Commission is still optimistic about reaching the target. “We can deliver the 70% if companies deliver on our agreements. The targets set out by the Commission are ambitious – because that is what we need in order to achieve sufficient protection for everyone in this pandemic.”
Meeting these targets does of course not only depend on member states, but also on the approval of additional safe and effective vaccines as well as the delivery of the agreed doses within the timeframes agreed with the vaccine companies. Deliveries are crucial for the successful roll-out. The Commission stresses that the companies need to ensure that they fulfil their contractual obligations.
Two remaining questions are what the coverage, including the non-adult population, must be to ensure group immunity and if additional vaccination shots are required to boost the first two ones and take care of new variants of the virus.
M. Apelblat
The Brussels Times