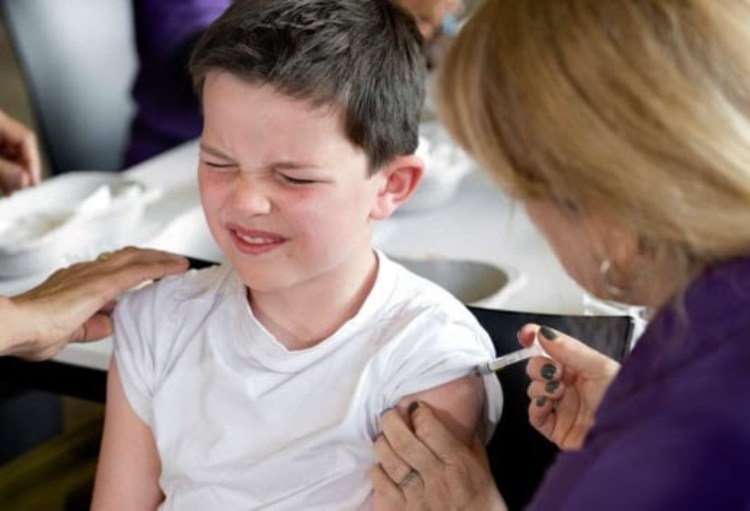
On Wednesday, the European Medicines Agency (EMA) has approved the Pfizer/BioNTech coronavirus vaccine for children aged 5 to 11 years, it announced.
The Agency recommends giving children between 5 and 11 a lower dose of the vaccine than is used in everyone aged 12 and over (10 µg compared with 30 µg). Like in the older age groups, it should also be administered as two injections in the muscles of the upper arm, three weeks apart.
The most common side effects in children under 11 years old are mild or moderate and similar to those in people aged 12 and above, and include pain at the injection site, tiredness, headache, redness and swelling at the site of injection, muscle pain and chills.
Therefore, the EMA concluded that the benefits of the vaccine for this young age group "outweigh the risks, particularly in those with conditions that increase the risk of severe Covid-19."
The EMA's advice will now go to the European Commission, which will decide whether the vaccine can actually be used for that age group in the EU.
If the Commission gives the official green light, Belgium and the other EU Member States can decide for themselves whether they will vaccinate children under the age of 12.
At the press conference following the Consultative Committee meeting last week, several ministers already indicated that Belgium wants to start this process as soon as possible.