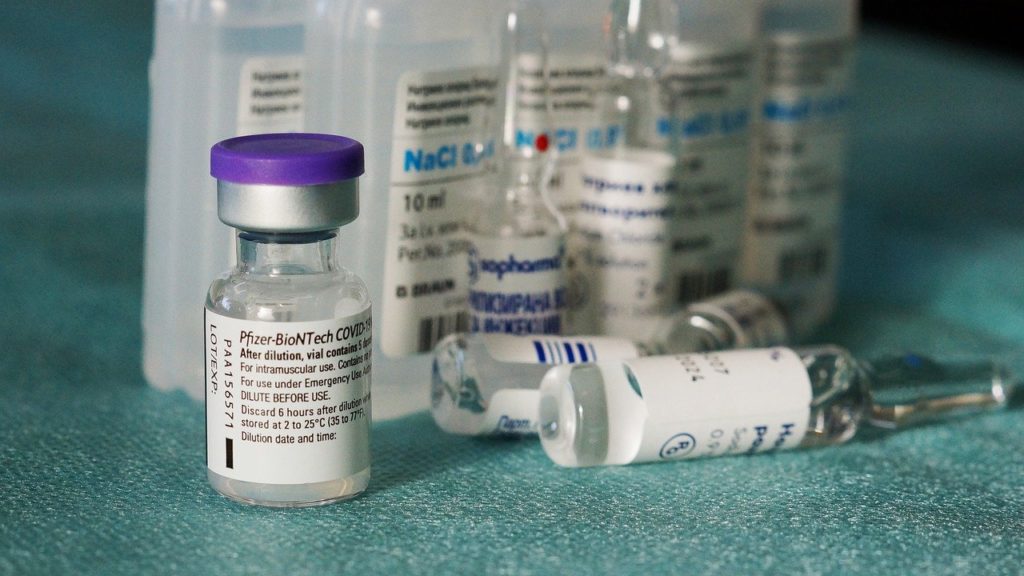
Some of the early batches of the Covid-19 vaccine produced by Pfizer/BioNTech – the first to be approved in Europe – contained lower levels of intact mRNA than expected, leaked documents have revealed.
A report on the problem has been published by the British Medical Journal (BMJ), which received some of the documents obtained illicitly from a hack of the computer system of the European Medicines Agency (EMA) that took place in December last year.
The hackers took possession of some 40Mb of data, which they then sent anonymously to journalists and academics.
“They came from anonymous email accounts and most efforts to interact with the senders were unsuccessful,” the BMJ reports. “None of the senders revealed their identity, and the EMA says it is pursuing a criminal investigation.”
In the meantime, the documents have been studied, and reveal some concerns around messenger RNA, the active ingredient of the new vaccines.
To quote the website of the Centers for Disease Control in the US: “mRNA vaccines are a new type of vaccine to protect against infectious diseases. To trigger an immune response, many vaccines put a weakened or inactivated germ into our bodies. Not mRNA vaccines,” the site explains.
“Instead, they teach our cells how to make a protein—or even just a piece of a protein—that triggers an immune response inside our bodies. That immune response, which produces antibodies, is what protects us from getting infected if the real virus enters our bodies.”
Related News
- People who don't get vaccinated will be infected 'sooner or later,' says Van Gucht
- EU will receive 4 million extra Pfizer vaccines by end of March
- 'Vaccines are key to freedom': Belgian PM takes stock of one year of pandemic
When EMA scientists studied the batches of vaccine Pfizer was proposing for commercial distribution, they found the mRNA present did not meet the manufacturer’s own specifications. And what is more, the implications of that discovery were not at all clear.
The differences ranged from 78% of healthy mRNA to only 55%, and the effect on the safety and efficacy of the vaccine as a result of the deterioration was, the EMA said, “yet to be defined”.
The EMA approved the Pfizer vaccine on 21 December, by which time its misgivings had been resolved enough for it to publish in its public assessment report that “the quality of this medicinal product, submitted in the emergency context of the current (covid-19) pandemic, is considered to be sufficiently consistent and acceptable.”
The BMJ speculates, based on another email from the US, that rising percentages of intact mRNA were behind the decision to approve.
This, the journal writes, raises a number of issues on a series of extremely technical matters surrounding the safety and effectiveness of the new generation of mRNA vaccines.
“Of particular concern is RNA instability, one of the most important variables relevant to all mRNA vaccines that has thus far received scant attention in the clinical community,” the journal states.
“It is an issue relevant not just to Pfizer-BioNTech vaccine but also to those produced by Moderna, CureVac, and others, as well as a ‘second generation’ mRNA vaccine being pursued by Imperial College London.”
Alan Hope
The Brussels Times