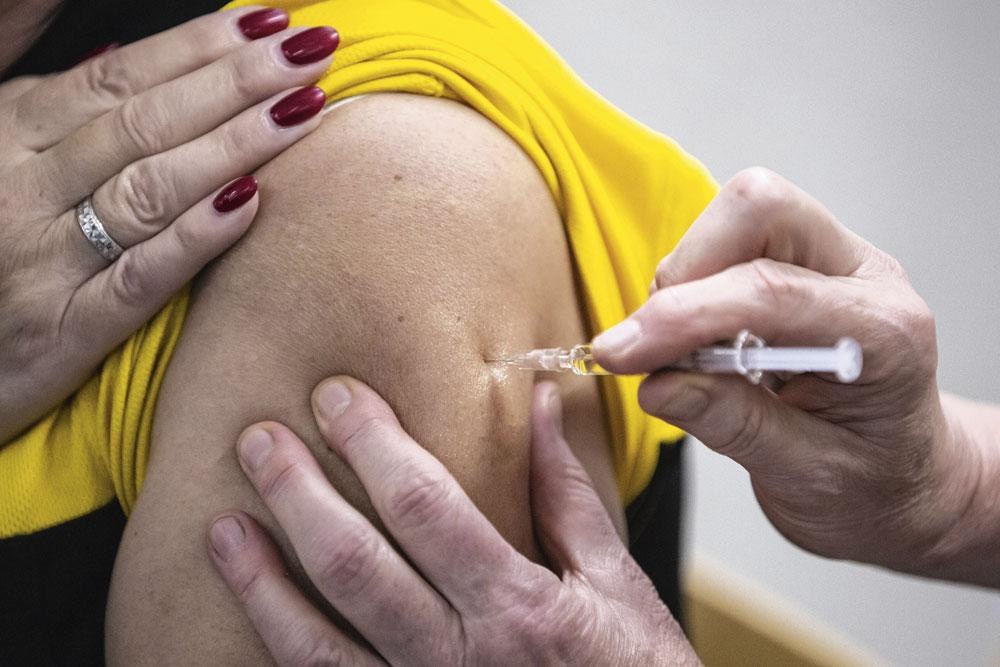
At least 400 people will be vaccinated at Ghent University Hospital (UZ Gent) with Curevac's Covid-19 vaccine as it progresses to the third and final phase of clinical trials.
The vaccine was already being tested at UZ Gent, but from Monday, a larger group of people will get the vaccine administered as part of Curevac's phase 3 trial, which should involve more than 35,000 people across Europe and Latin America.
The Curevac vaccine is one of several being developed to keep people from getting infected with Covid-19. A vaccine by Pfizer and BioNTech has already received approval in the UK and the United States, but no vaccine has been approved in Europe yet.
The European Medicines Agency (EMA) is meeting on Monday to discuss the Pfizer - BioNTech vaccine’s authorisation in the EU.
Related News
- European vaccine prices revealed in Belgian Twitter blunder
- Curevac launches third clinical trial phase for Covid-19 vaccine
- Europe set to approve Pfizer's Covid-19 vaccine before Christmas
If EMA recommends a marketing authorisation, the Commission will fast track its decision-making process with a view to granting a marketing authorisation valid in all EU and EEA Member States within days.
Assuming EMA approves the Pfizer vaccine, vaccination can start across the EU on 27, 28 and 29 December, as European Commission President Ursula von der Leyen tweeted on Thursday.
Belgium’s Health Minister Frank Vandenbroucke confirmed that Belgium is ready to start administering its first vaccines before the end of the year “if Pfizer can evenly distribute a first symbolic shipment in all European countries.”
In any case, “several vaccines are necessary to reach the world’s population,” commented Isabel Leroux-Roels, head of the Centre for Vaccinology at UZ Gent. “The passage of this trial into phase 3 is therefore good news for the development and possible approval of other vaccines against Covid-19.”
The Curevac vaccine is one of several ordered by Belgium provided it receives all the necessary authorisations.
Jason Spinks
The Brussels Times